Medecins Sans Frontieres (MSF), which has taken sides against Novartis in this issue, will make a presentation on how the issue will adversely affect millions of patients.
MUMBAI: A hearing on the simmering tussle between Swiss pharma major Novartis and the Indian government over the latter’s rejection of patent to cancer drug Gleevec is slated in the European Union parliament on Tuesday.
Medecins Sans Frontieres (MSF), which has taken sides against Novartis in this issue, will make a presentation on how the issue will adversely affect millions of patients. The patent was rejected under section 3 (d) of Indian patent law. Under the section, any new use or a new variant of an old substance cannot be patented unless it is found to have significant improved efficacy.
In January 2006, Novartis contended that section 3(d) of the Indian Patent Act does not conform to the WTO Agreement on Trade Related Aspects of Intellectual Property Rights (TRIPS). This was following the rejection of its patent application for Gleevec (imatinib mesylate) by the Chennai patent office on the grounds that it was not an innovation.
“Novartis is challenging the rights of millions of patients for affordable medicines by challenging section 3 (d) of Indian Patent Act. If the monopoly of a drug continues by making trivial changes, it will severely affect millions of patients who depend on Indian industry for cheaper versions of drugs,” Leena Menghaney, MSF campaigner, India, said.
Novartis sells the $2-billion blood cancer drug, Gleevec, at Rs 14.4 lakh per patient per year, while generic versions of the drug are available in India for about Rs 96,000 ($2100) per patient per year.
“Our case in India is solely about safeguarding intellectual property, not about patients’ access to medicines. Novartis is not challenging any provisions of the Indian patent law that were put in place to promote access. In fact, Novartis supports the TRIPS conditions that promote access for developing countries,” Ranjit Shahni, managing director, Novartis India, said.
![submenu-img]() Meet Isha Ambani's lesser-known relative who owns Rs 6368 crore business, Mukesh Ambani is his...
Meet Isha Ambani's lesser-known relative who owns Rs 6368 crore business, Mukesh Ambani is his...![submenu-img]() India gets a new supercar with 325 km/h top speed, priced at Rs 3.99 crore, it is made by…
India gets a new supercar with 325 km/h top speed, priced at Rs 3.99 crore, it is made by…![submenu-img]() Akash Ambani led Reliance Jio creates a new record, it is now world’s largest…
Akash Ambani led Reliance Jio creates a new record, it is now world’s largest…![submenu-img]() Hema Malini reveals Dharmendra was against her political career: ‘He told me…’
Hema Malini reveals Dharmendra was against her political career: ‘He told me…’ ![submenu-img]() US Congress passes Ukraine, Israel foreign aid bill worth $95 billion
US Congress passes Ukraine, Israel foreign aid bill worth $95 billion![submenu-img]() DNA Verified: Is CAA an anti-Muslim law? Centre terms news report as 'misleading'
DNA Verified: Is CAA an anti-Muslim law? Centre terms news report as 'misleading'![submenu-img]() DNA Verified: Lok Sabha Elections 2024 to be held on April 19? Know truth behind viral message
DNA Verified: Lok Sabha Elections 2024 to be held on April 19? Know truth behind viral message![submenu-img]() DNA Verified: Modi govt giving students free laptops under 'One Student One Laptop' scheme? Know truth here
DNA Verified: Modi govt giving students free laptops under 'One Student One Laptop' scheme? Know truth here![submenu-img]() DNA Verified: Shah Rukh Khan denies reports of his role in release of India's naval officers from Qatar
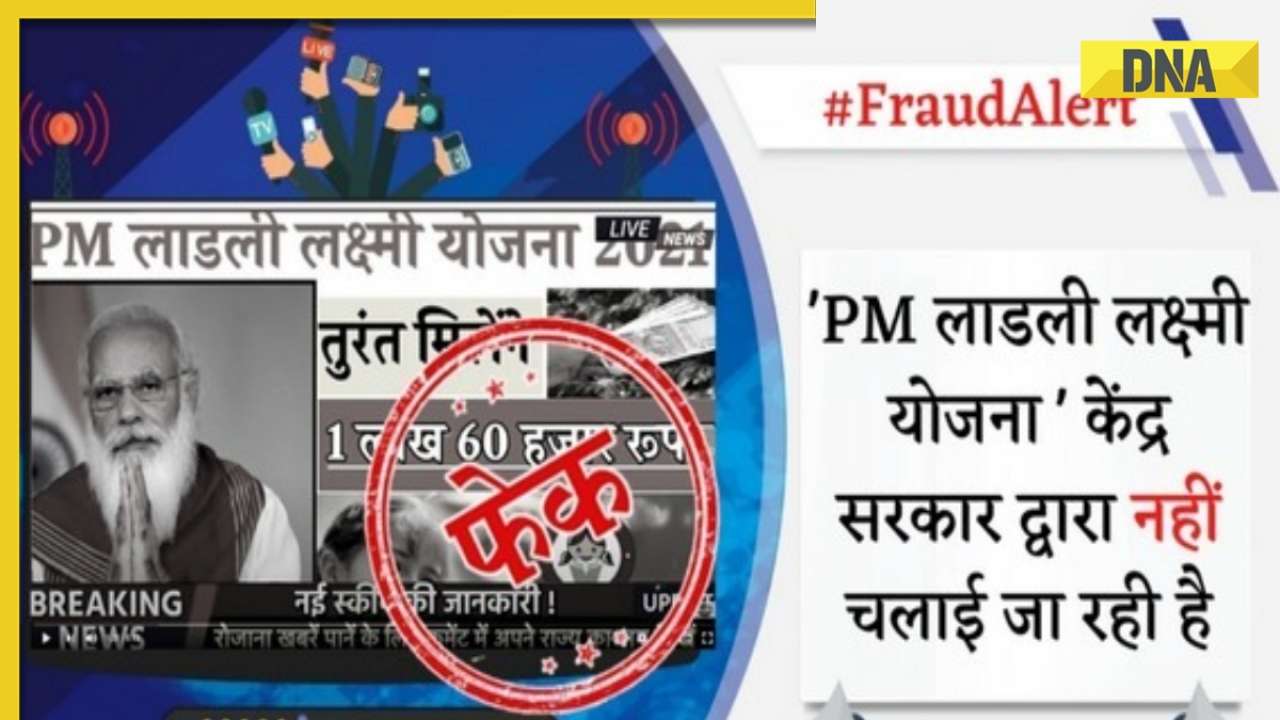
DNA Verified: Shah Rukh Khan denies reports of his role in release of India's naval officers from Qatar![submenu-img]() DNA Verified: Is govt providing Rs 1.6 lakh benefit to girls under PM Ladli Laxmi Yojana? Know truth
DNA Verified: Is govt providing Rs 1.6 lakh benefit to girls under PM Ladli Laxmi Yojana? Know truth![submenu-img]() Streaming This Week: Crakk, Tillu Square, Ranneeti, Dil Dosti Dilemma, latest OTT releases to binge-watch
Streaming This Week: Crakk, Tillu Square, Ranneeti, Dil Dosti Dilemma, latest OTT releases to binge-watch![submenu-img]() From Salman Khan to Shah Rukh Khan: Actors who de-aged for films before Amitabh Bachchan in Kalki 2898 AD
From Salman Khan to Shah Rukh Khan: Actors who de-aged for films before Amitabh Bachchan in Kalki 2898 AD![submenu-img]() Remember Abhishek Sharma? Hrithik Roshan's brother from Kaho Naa Pyaar Hai has become TV star, is married to..
Remember Abhishek Sharma? Hrithik Roshan's brother from Kaho Naa Pyaar Hai has become TV star, is married to..![submenu-img]() Remember Ali Haji? Aamir Khan, Kajol's son in Fanaa, who is now director, writer; here's how charming he looks now
Remember Ali Haji? Aamir Khan, Kajol's son in Fanaa, who is now director, writer; here's how charming he looks now![submenu-img]() Remember Sana Saeed? SRK's daughter in Kuch Kuch Hota Hai, here's how she looks after 26 years, she's dating..
Remember Sana Saeed? SRK's daughter in Kuch Kuch Hota Hai, here's how she looks after 26 years, she's dating..![submenu-img]() DNA Explainer: What is cloud seeding which is blamed for wreaking havoc in Dubai?
DNA Explainer: What is cloud seeding which is blamed for wreaking havoc in Dubai?![submenu-img]() DNA Explainer: What is Israel's Arrow-3 defence system used to intercept Iran's missile attack?
DNA Explainer: What is Israel's Arrow-3 defence system used to intercept Iran's missile attack?![submenu-img]() DNA Explainer: How Iranian projectiles failed to breach iron-clad Israeli air defence
DNA Explainer: How Iranian projectiles failed to breach iron-clad Israeli air defence![submenu-img]() DNA Explainer: What is India's stand amid Iran-Israel conflict?
DNA Explainer: What is India's stand amid Iran-Israel conflict?![submenu-img]() DNA Explainer: Why Iran attacked Israel with hundreds of drones, missiles
DNA Explainer: Why Iran attacked Israel with hundreds of drones, missiles![submenu-img]() Hema Malini reveals Dharmendra was against her political career: ‘He told me…’
Hema Malini reveals Dharmendra was against her political career: ‘He told me…’ ![submenu-img]() Meet diva sister of Bollywood star, who served as lieutenant in Indian Army; she is now working as...
Meet diva sister of Bollywood star, who served as lieutenant in Indian Army; she is now working as...![submenu-img]() Meet actor, who worked as background dancer, once had only Rs 20 in pocket; now earns Rs 10 crore per film, is worth...
Meet actor, who worked as background dancer, once had only Rs 20 in pocket; now earns Rs 10 crore per film, is worth...![submenu-img]() Ranveer Singh's biggest flop suffered Rs 90-crore loss, faced several delays, was remake of 80s' classic, earned only...
Ranveer Singh's biggest flop suffered Rs 90-crore loss, faced several delays, was remake of 80s' classic, earned only...![submenu-img]() Fahadh Faasil shares how Malayalam cinema's business model is different: 'Unlike the rest of India...'
Fahadh Faasil shares how Malayalam cinema's business model is different: 'Unlike the rest of India...'![submenu-img]() IPL 2024: Marcus Stoinis' century power LSG to 6-wicket win over CSK
IPL 2024: Marcus Stoinis' century power LSG to 6-wicket win over CSK![submenu-img]() DC vs GT, IPL 2024: Predicted playing XI, live streaming details, weather and pitch report
DC vs GT, IPL 2024: Predicted playing XI, live streaming details, weather and pitch report![submenu-img]() DC vs GT IPL 2024 Dream11 prediction: Fantasy cricket tips for Delhi Capitals vs Gujarat Titans
DC vs GT IPL 2024 Dream11 prediction: Fantasy cricket tips for Delhi Capitals vs Gujarat Titans ![submenu-img]() IPL 2024: How can MI still qualify for playoffs after 9-wicket loss against RR?
IPL 2024: How can MI still qualify for playoffs after 9-wicket loss against RR?![submenu-img]() IPL 2024: Yashasvi Jaiswal, Sandeep Sharma guide Rajasthan Royals to 9-wicket win over Mumbai Indians
IPL 2024: Yashasvi Jaiswal, Sandeep Sharma guide Rajasthan Royals to 9-wicket win over Mumbai Indians![submenu-img]() This city to witness 'Zero Shadow Day' today; know everything about this rare celestial event
This city to witness 'Zero Shadow Day' today; know everything about this rare celestial event![submenu-img]() Woman diagnosed with 'love brain' after calling boyfriend 100 times daily, details inside
Woman diagnosed with 'love brain' after calling boyfriend 100 times daily, details inside![submenu-img]() Puppy reunites with mother after being stuck inside shop, viral video wins internet
Puppy reunites with mother after being stuck inside shop, viral video wins internet![submenu-img]() Meet man, an Indian, who made changes in his super expensive Rolls Royce for comfort of his...
Meet man, an Indian, who made changes in his super expensive Rolls Royce for comfort of his...![submenu-img]() Mukesh Ambani's son Anant Ambani likely to get married to Radhika Merchant in July at…
Mukesh Ambani's son Anant Ambani likely to get married to Radhika Merchant in July at…












































)
)
)
)
)
)