Experts expressed their concern over the lack of research culture in Indian medical colleges and hospitals. They called for an interface between the academia and industry.
Experts and investors at the Bangalore India Bio-2010 on Wednesday made a strong plea for a strong interface between the academia and industry to enable the country to grab huge opportunities in Biotechnology research, especially in clinical trials.
Dr Moni Abraham Kuriakose from Narayana Hrudayalaya, participating in a panel discussion on the issue, said, “India has highly trained work force, world-class health facilities, 221 medical colleges and a huge cost advantage in clinical trials. But still, the potential has not been fully utilised.”
India has 50% to 75% cost advantage in clinical trials and “we have a great opportunity to bag a huge share of the $26 billion clinical trials market in the world. India has just about 500 to 1,000 clinical investigators and it is too small, when compared with countries like the USA which has 50,000 of them. India is training about 1,000 clinical investigators each year and it should be ramped up to at least 10,000,” he said.
Dr Kuriakose wanted the government and private sector to initiate measures to develop research culture in medical colleges and hospitals, and encourage research as a career.
Dr Ravi Kumar Banda, managing director of Xcyton Diagnostics Ltd, said “There are large gaps between the industry, academia and investor that need to be bridged. Institutes and research facilities should develop technology platforms or proof of concept for a product to showcase to the industry. Most collaborations fail because of the way the academia approach the industry. Some of the academic institutions like the Indian Institute of Science have good infrastructure. But not even 20% of its potential is being used for research and development.”
Prof Gayatri Saberwal, scientific officer, Institute of Bioinformatics and Applied Biotechnology (IBAB), said “75% of the students passed out from IBAB have been placed in premier organisations such as Biocon, BrickWood, IBM, etc. IBAB is a novel private academic institute of the Govt of Karnataka and ICICI Bank. One of the challenges faced by the institute is the funding scheme of the Central government.”
Dr SR Rao, adviser, Department of Biotechnology under the Central government, said, “There is very little institutionalised relationship between industry and universities and only about 1,500 projects have been funded so far. The barriers in linkages and partnership are lack of mutual trust and appreciation, lack of financial gains, lack of infrastructure facilities, lack of frame work and different norms of evaluation.”
Dr Sunny Sharma, senior managing director (Asia), OrbiMed Advisors, India Pvt., Ltd., said, “The role of venture capital in life sciences is very much needed. Access of capital is important for research and development. What OrbiMed looks for are novel products and technology, technological breakthrough, strong management team, and growing market. Investors like OrbiMed can help in certain areas such as providing a long-term, value-added, strategic source of capital helping management achieve strategic, financial and operational objectives via participation at the board level.”
![submenu-img]() US imposes sanctions on Chinese, Belarus firms for providing ballistic missile tech to Pakistan
US imposes sanctions on Chinese, Belarus firms for providing ballistic missile tech to Pakistan![submenu-img]() 'Don't have any comment': White House mum on reports of Israeli strikes in Iran
'Don't have any comment': White House mum on reports of Israeli strikes in Iran![submenu-img]() Yes Bank co-founder Rana Kapoor gets bail after four years in bank fraud case
Yes Bank co-founder Rana Kapoor gets bail after four years in bank fraud case![submenu-img]() Barmer Lok Sabha Polls 2024: Check key candidates, date of voting and other important details
Barmer Lok Sabha Polls 2024: Check key candidates, date of voting and other important details![submenu-img]() This star once lived in garage, earned Rs 51 as first salary; now charges Rs 5 crore per film, is worth Rs 335 crore
This star once lived in garage, earned Rs 51 as first salary; now charges Rs 5 crore per film, is worth Rs 335 crore![submenu-img]() DNA Verified: Is CAA an anti-Muslim law? Centre terms news report as 'misleading'
DNA Verified: Is CAA an anti-Muslim law? Centre terms news report as 'misleading'![submenu-img]() DNA Verified: Lok Sabha Elections 2024 to be held on April 19? Know truth behind viral message
DNA Verified: Lok Sabha Elections 2024 to be held on April 19? Know truth behind viral message![submenu-img]() DNA Verified: Modi govt giving students free laptops under 'One Student One Laptop' scheme? Know truth here
DNA Verified: Modi govt giving students free laptops under 'One Student One Laptop' scheme? Know truth here![submenu-img]() DNA Verified: Shah Rukh Khan denies reports of his role in release of India's naval officers from Qatar
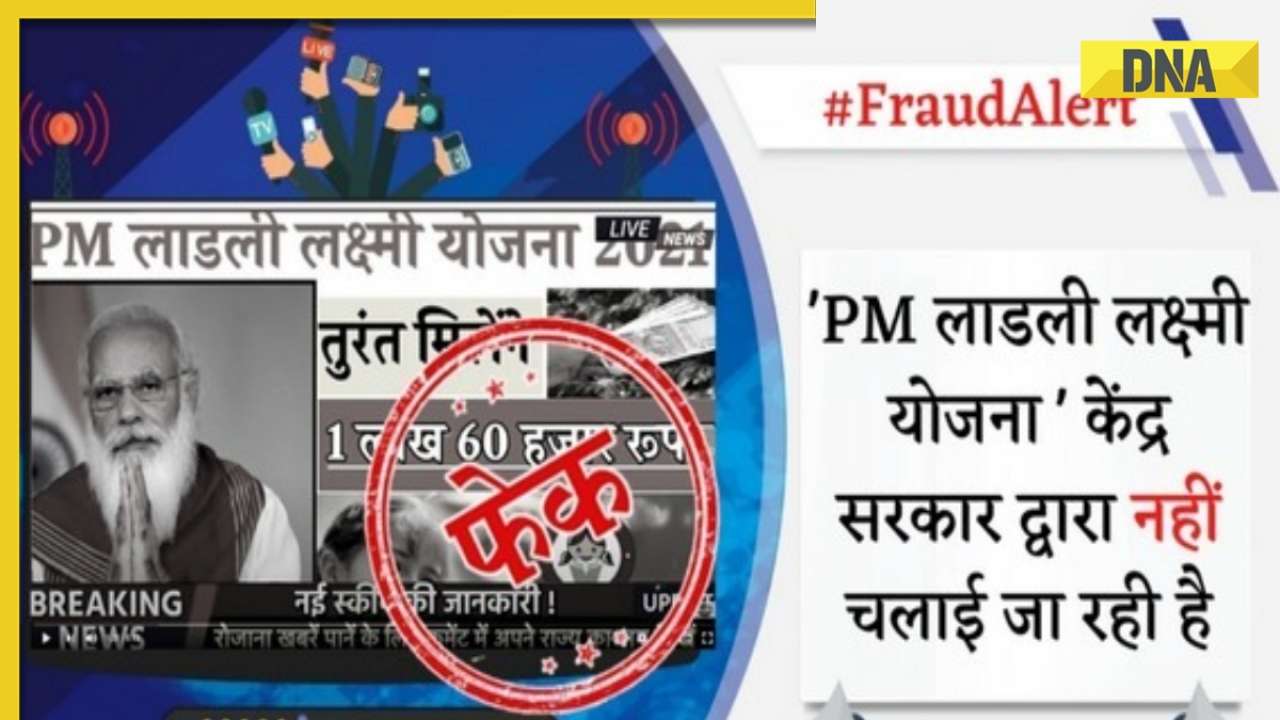
DNA Verified: Shah Rukh Khan denies reports of his role in release of India's naval officers from Qatar![submenu-img]() DNA Verified: Is govt providing Rs 1.6 lakh benefit to girls under PM Ladli Laxmi Yojana? Know truth
DNA Verified: Is govt providing Rs 1.6 lakh benefit to girls under PM Ladli Laxmi Yojana? Know truth![submenu-img]() Remember Ali Haji? Aamir Khan, Kajol's son in Fanaa, who is now director, writer; here's how charming he looks now
Remember Ali Haji? Aamir Khan, Kajol's son in Fanaa, who is now director, writer; here's how charming he looks now![submenu-img]() Remember Sana Saeed? SRK's daughter in Kuch Kuch Hota Hai, here's how she looks after 26 years, she's dating..
Remember Sana Saeed? SRK's daughter in Kuch Kuch Hota Hai, here's how she looks after 26 years, she's dating..![submenu-img]() In pics: Rajinikanth, Kamal Haasan, Mani Ratnam, Suriya attend S Shankar's daughter Aishwarya's star-studded wedding
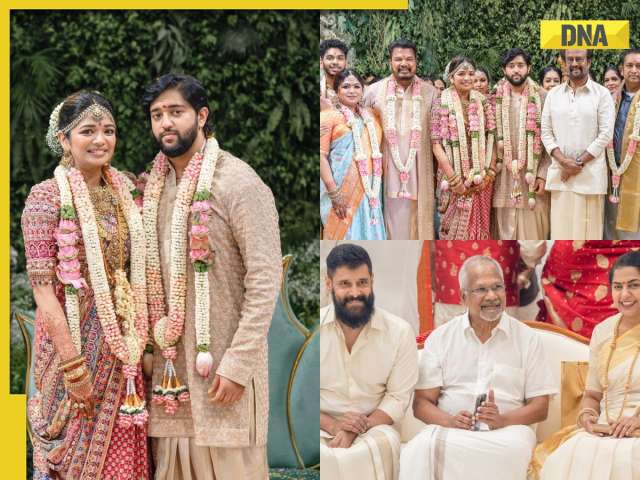
In pics: Rajinikanth, Kamal Haasan, Mani Ratnam, Suriya attend S Shankar's daughter Aishwarya's star-studded wedding![submenu-img]() In pics: Sanya Malhotra attends opening of school for neurodivergent individuals to mark World Autism Month
In pics: Sanya Malhotra attends opening of school for neurodivergent individuals to mark World Autism Month![submenu-img]() Remember Jibraan Khan? Shah Rukh's son in Kabhi Khushi Kabhie Gham, who worked in Brahmastra; here’s how he looks now
Remember Jibraan Khan? Shah Rukh's son in Kabhi Khushi Kabhie Gham, who worked in Brahmastra; here’s how he looks now![submenu-img]() DNA Explainer: What is cloud seeding which is blamed for wreaking havoc in Dubai?
DNA Explainer: What is cloud seeding which is blamed for wreaking havoc in Dubai?![submenu-img]() DNA Explainer: What is Israel's Arrow-3 defence system used to intercept Iran's missile attack?
DNA Explainer: What is Israel's Arrow-3 defence system used to intercept Iran's missile attack?![submenu-img]() DNA Explainer: How Iranian projectiles failed to breach iron-clad Israeli air defence
DNA Explainer: How Iranian projectiles failed to breach iron-clad Israeli air defence![submenu-img]() DNA Explainer: What is India's stand amid Iran-Israel conflict?
DNA Explainer: What is India's stand amid Iran-Israel conflict?![submenu-img]() DNA Explainer: Why Iran attacked Israel with hundreds of drones, missiles
DNA Explainer: Why Iran attacked Israel with hundreds of drones, missiles![submenu-img]() This star once lived in garage, earned Rs 51 as first salary; now charges Rs 5 crore per film, is worth Rs 335 crore
This star once lived in garage, earned Rs 51 as first salary; now charges Rs 5 crore per film, is worth Rs 335 crore![submenu-img]() Meet actress, who worked as cook for free food, mopped floors, one Instagram post changed her life, is now worth…
Meet actress, who worked as cook for free food, mopped floors, one Instagram post changed her life, is now worth… ![submenu-img]() UP man arrested for booking cab from Salman Khan's house under Lawrence Bishnoi's name
UP man arrested for booking cab from Salman Khan's house under Lawrence Bishnoi's name ![submenu-img]() 'Justice milega': Ankita Lokhande talks about Sushant Singh Rajput, reveals she's still connected with his family
'Justice milega': Ankita Lokhande talks about Sushant Singh Rajput, reveals she's still connected with his family![submenu-img]() Rajkummar Rao reacts to plastic surgery rumours, admits he got fillers: 'If something gives me confidence...'
Rajkummar Rao reacts to plastic surgery rumours, admits he got fillers: 'If something gives me confidence...'![submenu-img]() IPL 2024: KL Rahul, Quinton de Kock star in Lucknow Super Giants' dominating 8-wicket win over Chennai Super Kings
IPL 2024: KL Rahul, Quinton de Kock star in Lucknow Super Giants' dominating 8-wicket win over Chennai Super Kings![submenu-img]() DC vs SRH, IPL 2024: Predicted playing XI, live streaming details, weather and pitch report
DC vs SRH, IPL 2024: Predicted playing XI, live streaming details, weather and pitch report![submenu-img]() Watch: Virat Kohli's cheeky 'your wife' remark to Dinesh Karthik leaves RCB teammates in splits
Watch: Virat Kohli's cheeky 'your wife' remark to Dinesh Karthik leaves RCB teammates in splits ![submenu-img]() DC vs SRH IPL 2024 Dream11 prediction: Fantasy cricket tips for Delhi Capitals vs Sunrisers Hyderabad
DC vs SRH IPL 2024 Dream11 prediction: Fantasy cricket tips for Delhi Capitals vs Sunrisers Hyderabad![submenu-img]() 'Kohli said it's not an option, just...': KL Rahul recalls his IPL debut for RCB in 2013
'Kohli said it's not an option, just...': KL Rahul recalls his IPL debut for RCB in 2013![submenu-img]() Canada's biggest heist: Two Indian-origin men among six arrested for Rs 1300 crore cash, gold theft
Canada's biggest heist: Two Indian-origin men among six arrested for Rs 1300 crore cash, gold theft![submenu-img]() Donuru Ananya Reddy, who secured AIR 3 in UPSC CSE 2023, calls Virat Kohli her inspiration, says…
Donuru Ananya Reddy, who secured AIR 3 in UPSC CSE 2023, calls Virat Kohli her inspiration, says…![submenu-img]() Nestle getting children addicted to sugar, Cerelac contains 3 grams of sugar per serving in India but not in…
Nestle getting children addicted to sugar, Cerelac contains 3 grams of sugar per serving in India but not in…![submenu-img]() Viral video: Woman enters crowded Delhi bus wearing bikini, makes obscene gesture at passenger, watch
Viral video: Woman enters crowded Delhi bus wearing bikini, makes obscene gesture at passenger, watch![submenu-img]() This Swiss Alps wedding outshine Mukesh Ambani's son Anant Ambani's Jamnagar pre-wedding gala
This Swiss Alps wedding outshine Mukesh Ambani's son Anant Ambani's Jamnagar pre-wedding gala











































)
)
)
)
)
)